Drug Addictions: A Historical and Ethological Overview
1. Introduction
1.1. Preliminary considerations: Focus on cocaine and heroin
It is well established that several psychoactive substances can lead to addiction. These include legal drugs such as alcohol and nicotine which generate the major part of the addiction-related social and economical costs to modern societies, and a pleiad of illegal drugs amongst which cannabis, cocaine and heroin are the most commonly used.[1]
When one wants to consider the harmful consequences of an addictive drug, both the dependence and physical harm potencies of the drug should be considered for these two aspects contribute to the deterioration of the user’s life. A recent classification of the major classes of addictive drugs reveals that heroin and cocaine are clearly the most dangerous ones since both their addictive properties and physical harm potency are high.[2] Cocaine and heroin are followed by barbiturates and street methadone, but tobacco is shown to have addictive property of the same magnitude as cocaine, thereby demonstrating that the legal status of a substance is not a predictive factor of least addictive properties.
In the present chapter, we will consider exclusively cocaine and heroin addictions, not only because these two drugs are clearly the most dangerous ones, but mainly because cocaine and heroin use have been increasing among western countries populations in the last ten years. This focus is one limitation of the general conclusions that will be provided in the following chapters that will also address alcoholism and food addiction that will be joined by another addiction, namely pathological gambling, in the clinical definition of addictions in the upcoming DSM-V. Thus, addictions are increasingly recognized as abnormal persistent maladaptive behaviors driven by specific, initially reinforcing, stimuli in the environment that are not anymore restricted to psychoactive substances.
1.2 Drug use: A behavior as old as humankind?
Drug use seems to have entered human customs as early as the emergence of human societies. Evidences that recreational drug use has emerged early on after human sedentarisation, perhaps with the development of religious rites, can be found for several drugs and routes of administration.
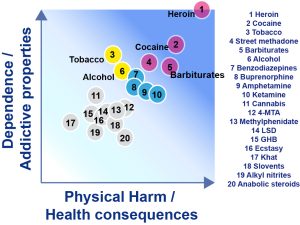
The addictive potential of a drug varies from substance to substance, and from individual to individual. Dose, frequency, pharmacokinetics of a particular substance, route of administration, and time are critical factors for physical harm and addictive potency. Heroin and cocaine are clearly the most dangerous ones since both their addictive properties and physical harm potency are high.
Thus, 5000 B.C. the Sumerians used opium, as suggested by the fact that they had an ideogram for it which has been translated as HUL, meaning “joy” or “rejoicing.”[3] A 3500 B.C. Egyptian papyrus provides the earliest historical record of the production of alcohol in the description of a brewery.[4]
Interestingly, 3000 B.C. is also the approximate date of the supposed origin of the use of tea in China. It is likely that coca leaf chewing began in the Andes at the same time since traces of coca have been found in mummies dating 3000 years back.[5] The cocaine content of coca leaf is under 1% but after 1859, when cocaine was first isolated from coca leaf by Albert Niemann, cocaine was available legally in concentrations that were nearly 100% pure. Cocaine was first used recreationally in the 1860s, almost as soon as it was synthesized. A few years after its synthesis by Richard Willstätter in 1898, cocaine appeared in cigarettes, ointments, nasal sprays, and tonics.[6] The most popular cocaine-based product was Mariani Wine (Vin Mariani). It was a wine and cocaine mixture that was launched in 1863. Nearly all popular personalities of the day, including Queen Victoria, Thomas Edison and Pope Leon XIII endorsed it. Cocaine has also been popularized by Sigmund Freud who prescribed it for the treatment of digestive disorders, asthma, depression or opiate and alcohol dependence.[7]
At the same time, more precisely in 1898, heroin (diacetylmorphine) was synthesized by Felix Hoffmann, 23 years after a first academic synthesis by Alder Wright. Akin to the launch of cocaine as a medicine, heroin was then introduced by Bayer as “safe preparation free from addiction-forming properties.”
The broad availability of the pure form of cocaine and heroin has contributed to the marked development of addiction to these substances which, in their primary forms and routes of administration, were far less addictive. This phenomenon has been suggested to stem from a discrepancy between our brain and our modern environment, i.e, Nesse and Berridge wrote in 1997: “We are vulnerable to such fitness-decreasing incentives because our brains are not designed to cope with ready access to pure drugs, video games, and snack foods. Hundreds of generations of exposure would likely shape resistance to their allure and their deleterious effects.”[8] This interesting consideration suggests that drug addiction may be a matter of mismatch between Human evolution and the recent revolution of human environment, a problem to which Evolution may be the best solution.
However, before these evolutionary, and rather fatalistic considerations, human societies have developed social and legal strategies to cope with addiction, as early as 10 years following the synthesis of heroin and cocaine. Indeed, the United States prohibited the importation of smoking opium[9] and the manufacture of heroin in 1909 and 1924, respectively, while the Harrison Narcotics Act of 1914 prohibited the use of cocaine. Since then law enforcement has limited, but not eradicated, heroin and cocaine use; there has been a general increase in cocaine use within European countries over the past 20 years. Such a trend may induce an increase in the prevalence of drug-related health problems, and most importantly, of drug addiction.
1.3 Drug use: An evolutionary feature of animal kingdom
Drug use seems inherent to animal behavior, perhaps because of the evolutionary selection of a reward system developed to maintain species survival, bringing animals towards sources of reinforcement. Thus spontaneous drug use has been observed in several species in the wild. Elephants would intoxicate with alcohol contained in ripe fruits and baboons would readily eat over-ripe fruits from the marula tree until they cannot walk anymore. Birds also use alcohol in that song thrush, for instance, struggle to fly after eating ripe grapes.
An exhaustive list of examples of spontaneous drug use in animal kingdom is beyond the scope of this chapter, but a last example should be enough to emphasize how broad are sources of intoxication in mammals: in the south of the United States, sheep and horses eat astragalus and then show hyperactive behavior akin to human beings.
In experimental settings, it has been demonstrated that all drugs abused by humans are reinforcing in many species including planarians[10] and flies,[11][12] and they are readily self-administered by vertebrates such as mice[13][14][15][16][17][18][19] or rats[20][21][22][23][24], dogs[25][26], and non human primates.[27][28]
Thus not only is drug used common to several species of the animal kingdom but the demonstration that pure forms of psychoactive drugs have reinforcing properties in animals under experimental conditions suggests that drug taking is not a specific behavioral feature of human beings. Drug use in animals seems rather to be the evidence that the neurobiological substrates of primary motivational and reinforcement processes selected by evolution have been shaped early on and maintained from planarians to human beings, and that drugs highjack these systems.
However, it remains unclear the extent to which these findings help inform our understanding of drug addiction in humans since it is a brain disorder that is clearly far removed from primary reinforcement mechanisms.
2. Drug addiction: A human-specific disorder?
2.1 What is drug addiction?
Drug addiction is a complex brain disorder,[29] affecting the motivational,[30][31] learning[32][33][34][35] and behavioral control systems of the brain.[36][37][38] Several definitions of drug addiction, ranging from the psychiatric to the social view have been presented by Koob and Le Moal[39] and will not be discussed any further.
Drug addiction is defined as a chronic relapsing compulsive habit characterized by loss of control over drug intake, maintained drug use despite adverse consequences[40][41][42] and the development of negative psycho-affective distress when access to the drug is prevented.[43][44]
Because the aetiology and pathophysiology of drug addiction remain unknown, this prominent psychiatric disorder is best defined by the clinical features of the DSM-IV[45] (Figure 2). The diagnostic of drug addiction is currently based on a categorial dichotomous approach in that the patient must present at least three out of the seven clinical criteria listed in figure 5 to be said addicted to a substance.
Fig. 2. Clinical features of drug addiction according to the DSM-IV-R[46]
- Need for markedly increased amounts of a substance to achieve intoxication or desired effect, or markedly diminished effect with continued use of the same amount of the substance.
- The presence of a characteristic withdrawal syndrome or use of a substance (or closely related substance) to relieve or avoid withdrawal symptoms.
- Persistent desire to use drugs or one or more unsuccessful efforts to cut down or control substance.
- Substance used in larger amounts or over a longer period of time than the person intended.
- Important social, occupational, or recreational activities given up or reduced because of substance use.
- A great deal of time spent in activities necessary to obtain, to use or to recover from the effects of substance used.
- Continued substance use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to be caused or exacerbated by continued use.
The subject is diagnosed addicted to the substance if they show at least three out of the 7 clinical criteria over the last 12 months.
However, all addicted patients are not equally severely affected and a dimensional addiction severity scale has been developed to assess general behavioral, health and social drug-induced impairments.[47][48][49][50][51]
Indeed, drug addicts do not only take drugs, they spend great amounts of time foraging for their drugs, compulsively take drugs, lose control over drug intake, and persist in taking drugs despite the many adverse consequences of doing so, including compromising their health, family relationships, friendships and work. Many drug addicts resort to criminal behavior to obtain the funds necessary to sustain their compulsive drug use and the great majority eventually relapse to drug use even after prolonged periods of abstinence.
This negative behavioral picture illustrates how drug addiction is not merely a drug taking disorder. Indeed, among the individuals exposed to drugs, and there are many who occasionally drink only a glass or two of an alcoholic beverage, or smoke a cigarette or two, only 15 to 30% overall will switch from casual, ‘recreational’ drug use to drug abuse and drug addiction[52][53] (Figure 3).
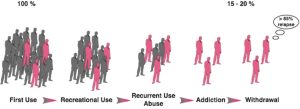
A substantial proportion of the general population experiences drugs at least once in a lifetime. Of the recreational users who control their drug intake, some will shift to more chronic drug use. Only a subgroup of these individuals will develop drug abuse and eventually drug addiction. Epidemiological studies reveal that of the individuals who have been exposed to addictive drugs, 15 to 20 % eventually develop addiction.
Despite considerable research we still do not understand why some individuals develop a compulsive use of drugs nor do we have effective treatments[54] to reduce the substantial social and economic burden.[55] Nevertheless, there is increasing evidence suggesting that drug addiction results from gradual adaptation processes in the brain of vulnerable subjects in response to chronic drug exposure. Not only do these between-systems adaptations trigger an emotional allostatic state (hedonic allostasis)[56][57][58][59] characterized for instance by increased anxiety, irritability and depression but they may ultimately lead to a shift in the psychological mechanisms that govern drug seeking and drug taking behaviors, including habits[60][61][62][63][64][65] as aberrant instrumental learning mechanisms controlled by Pavlovian cues, altered behavioral control,[66][67][68][69] decision-making and self-monitoring processes.[70]
Similarly, Everitt and colleagues have argued that, during the development of drug addiction, drug seeking is initially goal-directed but becomes habitual, and ultimately compulsive, thereby emphasizing the potential importance of maladaptive automatic instrumental learning mechanisms and their control by Pavlovian incentive processes, so called incentive habits,[71][72] in the emergence of compulsive drug use.[73][74][75][76] Additionally, drug-induced adaptations may also facilitate the shift from impulsivity to compulsivity that has been suggested to occur in the development of drug addiction (Figure 6)[77] whereby only vulnerable subjects would show a transition from impulse-related recreational drug use to compulsive drug intake.[78]
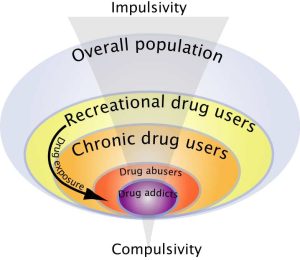
2.2 Behavioral and psychological profile of drug addicts
Besides their disinterest for alternative sources of reinforcement and their focus on the drug, drug addicts are characterized by several behavioral and cognitive deficits including impaired inhibitory control,[79][80][81][82][83][84] decision making[85][86][87][88][89][90][91][92] and insight.[93][94][95]
However, major differences can be observed between addicts depending on their preferred drug of abuse. For instance, although opiate and stimulant addicts both display increased sensation seeking[96][97][98] and impulsivity,[99][100][101][102][103][104] they nevertheless differ in other respects, with heroin addicts showing greater anxiety than cocaine addicts,[105] while the latter display higher impulsivity.[106][107][108]
Thus not only are several personality traits, including sensation seeking, anxiety and impulsivity, associated with increased vulnerability to use drugs (91-94),[109][110][111][112] but different personality traits are preferentially associated with use[113] and addiction to specific drugs.[114][115][116][117][118][119][120][121][122][123][124] It is therefore possible that heroin and cocaine addicts may self-medicate different personality characteristics or affective states,[125][126][127][128] with impulsivity being preferentially self-medicated by cocaine use. However, the relative contribution of a behavioral trait to the choice of a drug does not necessarily predict its implication in the transition to compulsive drug use.
Drug addicts also show several comorbid psychiatric disorders (108-111),[129][130][131][132] as stated by O’Brien: “Psychiatric disorders commonly coexist with addictive disorders. These include anxiety disorders, psychotic disorders, and affective disorders such as depression. Although some of these so-called ‘dual diagnosis’ cases are simply a coincidental occurrence of common disorders, the overlap is greater than would be expected by chance on the basis of population prevalences.”[133][134] However, it remains unknown whether comorbid elements contribute to increased vulnerability to drug addiction[135] or whether chronic drug exposure facilitates the emergence of psychiatric comorbidity.[136] Similarly, while some personality, or behavioral, traits are triggered by chronic drug use, there is evidence that personality variables are associated with increased vulnerability to develop drug addiction.[137][138] This rather blur picture not only suggests that several sub-populations exist within drug addicts,[139] but it clearly illustrates how little is known about the factors involved in the vulnerability to develop drug addiction.
To date a triadic model of contributing factors has been established that accounts well for both clinical and preclinical literature. Thus, vulnerability to drug addiction is suggested to result from the interaction between a vulnerable phenotype, or personality (being the interaction between genes and history), the drug and the environment (Figure 5).
There is clearly a genetic vulnerability to addiction. Genetic factors may contribute up to 40% to the development of drug addiction.[140] This estimation gives genetic factors a limited contribution to the vulnerability to drug addiction and highlights the importance of both the drug and the environment in the development of the pathology. There is indeed compelling evidence that life experiences and environments highly influence the effects of drugs of abuse and play a critical role in the transition from controlled to compulsive drug use.[141][142] For instance, drug addiction seems to be more frequent in people living in degraded areas or in people that undergo difficult experiences during their childhood. Such specific environmental conditions at either perinatal, developmental or adulthood stages may alter one’s personality construction so that they become more vulnerable to use or abuse drugs.[143] On the other hand, positive family relationships, friendships, involvement and attachment appear to somehow protect against the development of drug addiction (119, 120).[144][145]
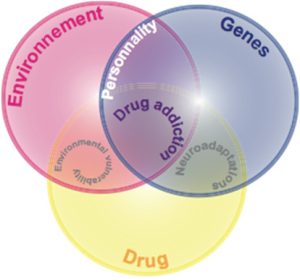
A number of interacting factors are hypothesized to influence the pathway to addiction, including biological determinants (genes), drug exposure and the environment. Genetic influences may account for up to 40% of the vulnerability for drug addiction.
Thus, the present general strategies developed to treat addictions should perhaps be reoriented towards a more patient-based medication strategy once better insights are gained in the understanding of the etiological and neurobiological substrates of individual vulnerabilities to addictions.
2.3 Biological correlates of drug addiction in humans: Insights from imaging studies
An exhaustive synthesis of the neurobiological correlates of drug addiction is beyond the scope of this chapter. Overall, drug exposure impacts both brain structure and function. Thus at the morphological level, drug addicts have decreased grey matter volumes in prefrontal[146][147][148][149][150] and cerebellar regions of the brain.[151] Functionally, when presented with drug-related cues that induce craving, drug addicts show abnormal activation of limbic structures including the amygdala,[152][153] the insular[154][155][156] and orbitofrontal cortices[157][158] as well as cognitive prefrontal areas such as the cingulate[159][160][161] and dorsolateral prefrontal cortices.[162]
Moreover, drug addicts are characterized by decreased levels of striatal D2/3 dopamine receptors[163][164][165] and reduced metabolism in the orbitofrontal cortex.[166] These two alterations are highly correlated,[167] thereby providing the orbitofrontal-limbic striatum circuit a prominent implication in addiction (134, 135),[168][169] even though other networks, including the thalamo-cortical systems, have been identified to be impaired in drug addicts.[170]
Interestingly, a growing body of evidence points towards an implication of non limbic striatal areas in the pathophysiology of drug addiction since dopamine transmission is specifically increased in the dorsal striatum of cocaine addicts experiencing craving in response to presentation of drug-associated cues,[171][172] providing a neurobiological evidence for a progressive involvement of dorsal striatum-dependent habits[173][174][175] in drug addiction.[176][177][178][179][180]
A major limitation of human studies is that the data obtained, though clearly informative, are based on the comparison of current or former drug addicts and drug naive control subjects. Thereby, human studies cannot control for the effects of protracted drug exposure on the brain nor can they define whether the abnormalities observed in drug addicts are a pathological biological adaptation to drug exposure or predated drug use and hence are instead endophenotypes of vulnerability to drug addiction.
This is where the case for animal experimentation in addiction research is revealed compelling. Besides the aforementioned limitations, studies in human addicts are often prone to interpretative issues not least due to inter-subject variability in drug exposure, the frequent co-abuse of several drugs often in combination with alcohol, cannabis and nicotine, the regular occurrence of co-morbid brain disorders such as depression, conduct disorder and attention-deficit/hyperactivity disorder (ADHD) and the difficulty in controlling premorbid cognitive and intellectual abilities.
Citation
Belin-Rauscent, Aude, and David Beli. “Drug Addictions: An Historical and Ethological Overview.” Addictions – From Pathophysiology to Treatment, InTech, 19 Oct. 2012. Crossref, doi:10.5772/52165.
This is article is distributed under the terms of the Creative Commons Attribution 3.0 License (CC-BY).
- Koob G, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005; 8: 1442-1444. ↵
- Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007; 369: 1047-1053. ↵
- Lindesmith AR. Addiction and opiates. Chicago: Aldine Pub. Co.; 1968: vii, 295. ↵
- Fort J. The pleasure seekers; the drug crisis, youth, and society. Indianapolis: Bobbs-Merrill; 1969: 255. ↵
- Rivera MA, Aufderheide AC, Cartmell LW, Torres CM, Langsjoen O. Antiquity of coca-leaf chewing in the south central Andes: a 3,000 year archaeological record of coca-leaf chewing from northern Chile. J Psychoactive Drugs. 2005; 37: 455-458. ↵
- Humphrey AJ, O’Hagan D. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat Prod Rep. 2001; 18: 494-502. ↵
- Freud S. Uber coca. 1884; Centralbl Gesamte Ther: 289-314. ↵
- Nesse RM, Berridge K. Psychoactive Drug Use in Evolutionary Perspective. Science. 1997; 278: 63-66. ↵
- Kolb LC. Drug addiction. Bulletin of the New York Academy of Medicine. 1965; 41: 306. ↵
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000; 11: 2511-2513. ↵
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. CurrBiol. 2000; 10: 211-214. ↵
- Wolf F, Heberlein U. Invertebrate models of drug abuse. J Neurobiol. 2002; 54: 161-178. ↵
- Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991; 2: 477-480. ↵
- Grahame NJ, Phillips TJ, Burkhart-Kasch S, Cunningham CL. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol Biochem Behav. 1995; 51: 827-834. ↵
- Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl). 2002; 161: 417-424. ↵
- Cain ME, Denehy ED, Bardo M. Individual Differences in Amphetamine Self-Administration: The rôle of the Central Nucleus of the Amygdala. Neuropsychopharmacology. 2007; 33: 1149-1161. ↵
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behav Genet. 2007; 37: 101-118. ↵
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl). 2007; 193: 179-186. ↵
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009 ↵
- Pickens R, Harris WC. Self-administration of d-amphetamine by rats. Psychopharmacologia. 1968; 12: 158-163. ↵
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: Mediation by separate neural systems. Psychopharmacology. 1982; 78: 204-209. ↵
- Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl). 1984; 82: 6-13. ↵
- Koob G, Le HT, Creese I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. NeurosciLett. 1987; 79: 315-320. ↵
- Weissenborn R, Yackey M, Koob GF, Weiss F. Measures of cocaine-seeking behavior using a multiple schedule of food and drug self-administration in rats. Drug Alcohol Depend. 1995; 38: 237-246. ↵
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983; 224: 319-326. ↵
- Shannon HE, Risner ME. Comparison of behavior maintained by intravenous cocaine and d-amphetamine in dogs. JpharmacolExpTher. 1984; 229: 422-432. ↵
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969; 16: 30-48. ↵
- Goldberg SR, Woods JH, Schuster CR. Morphine: Conditioned Increases in Self-Administration in Rhesus Monkeys. Science. 1969 ↵
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997; 278: 45-47. ↵
- Robinson T, Berridge K. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000; 95: S91-S117. ↵
- Kalivas P, Volkow N. The Neural Basis of Addiction: A Pathology of Motivation and Choice. American Journal of Psychiatry. 2005; 162: 1403-1413. ↵
- Everitt B, Parkinson JA, Olmstead M, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann NYAcadSci. 1999; 877: 412-438. ↵
- Everitt BJ., Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research Reviews. 2001; 36: 129-138. ↵
- Everitt BJ., Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005; 8: 1481-1489. ↵
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ.. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009; 199(1): 89-102. ↵
- Volkow N, Fowler J. Addiction, a Disease of Compulsion and Drive: Involvement of the Orbitofrontal Cortex. Cerebral Cortex. 2000 ↵
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002; 159: 1642-1652. ↵
- Goldstein RZ, Alia-Klein N, Tomasi D et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009; 106: 9453-9458. ↵
- Koob G, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005; 8: 1442-1444. ↵
- Everitt BJ., Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005; 8: 1481-1489. ↵
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999; 398: 567-570. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, ACADEMIC PRESS; 2010. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, ACADEMIC PRESS; 2010. ↵
- Koob G, Le Moal M. Addiction and the Brain Antireward System. Annu Rev Psychol. 2008; 59: 29-53. ↵
- APA. Diagnostic and Statistical Manual of Mental Disorders fourth edition, Text revision (DSM-IV TR). Washington DC: American Psychiatric Association; 2000. ↵
- APA. Diagnostic and Statistical Manual of Mental Disorders fourth edition, Text revision (DSM-IV TR). Washington DC: American Psychiatric Association; 2000. ↵
- Mclellan A, Kushner H, Metzger D, Peters R. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992 ↵
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006; 15: 113-124. ↵
- Rikoon S, Cacciola J, Carise D, Alterman A, Mclellan A. Predicting DSM-IV dependence diagnoses from Addiction Severity Index composite scores. Journal of Substance Abuse Treatment. 2006; 31: 17-24. ↵
- Krenz S, Dieckmann S, Favrat B et al. French version of the addiction severity index (5th Edition): validity and reliability among Swiss opiate-dependent patients. French validation of the Addiction Severity Index. Eur Addict Res. 2004; 10: 173-179. ↵
- Cacciola J, Alterman A, O’Brien CP, Mclellan A. The Addiction Severity Index in clinical efficacy trials of medications for cocaine dependence. NIDA ResMonogr. 1997; 175: 182-191. ↵
- Koob G, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005; 8: 1442-1444. ↵
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National comorbidity Survey. Exp Clin Psychopharmacol. 1994; 2: 244-268. ↵
- Kreek M, Laforge K, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002; 1: 710-726. ↵
- Uhl G, Grow RW. The burden of complex genetics in brain disorders. Arch GenPsychiatry. 2004; 61: 223-229. ↵
- Koob G, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005; 8: 1442-1444. ↵
- Koob G, Moal ML. Drug Abuse: Hedonic Homeostatic Dysregulation. Science. 1997; 278: 52-58. ↵
- Koob G, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001; 24: 97-129. ↵
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008; 363: 3113-3123. ↵
- Everitt BJ., Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005; 8: 1481-1489. ↵
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ.. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009; 199(1): 89-102. ↵
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999; 398: 567-570. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, ACADEMIC PRESS; 2010. ↵
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: rôle of automatic and nonautomatic processes. Psychol Rev. 1990; 97: 147-168. ↵
- O’Brien CP, Mclellan A. Myths about the treatment of addiction. Lancet. 1996; 347: 237-240. ↵
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002; 159: 1642-1652. ↵
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999; 146: 373-390. ↵
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley J, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008; 363: 3125-3135. ↵
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008; 14: 185-189. ↵
- Baumeister RF, Heatherton TF, Tice DM. Losing Control: How and Why People Fail at Self-Regulation. 1994. ↵
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ.. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009; 199(1): 89-102. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, ACADEMIC PRESS; 2010. ↵
- Everitt BJ., Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research Reviews. 2001; 36: 129-138. ↵
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ.. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009; 199(1): 89-102. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, ACADEMIC PRESS; 2010. ↵
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley J, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008; 363: 3125-3135. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, Academic Press, 2010. ↵
- Koob G, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005; 8: 1442-1444. ↵
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004; 99: 461-471. ↵
- Mitchell C, Flaherty C. Differential effects of removing the glucose or saccharin components of a glucose–saccharin mixture in a successive negative contrast paradigm. Physiology & Behavior. 2005; 84: 579-583. ↵
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006; 12: 559-566. ↵
- Dom G, D’haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006; 101: 50-59. ↵
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007; 90: 2-11. ↵
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and biobehavioral reviews. 2008; 32: 777-810. ↵
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000; 38: 1180-1187. ↵
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001; 96: 1825-1837. ↵
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002; 40: 1675-1689. ↵
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002; 40: 1690-1705. ↵
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004; 24: 11017-11022. ↵
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005; 8: 1458-1463. ↵
- Verdejo-Garcia A, Vilar-Lopez R, Perez-Garcia M, Podell K, Goldberg E. Altered adaptive but not veridical decision-making in substance dependent individuals. J Int Neuropsychol Soc. 2006; 12: 90-99. ↵
- Vassileva J, Petkova P, Georgiev S et al. Impaired decision-making in psychopathic heroin addicts. Drug Alcohol Depend. 2007; 86: 287-289. ↵
- Goldstein RZ, Craig AD, Bechara A et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009; 13: 372-380. ↵
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009; 32: 56-67. ↵
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010. ↵
- Allcock CC, Grace DM. Pathological gamblers are neither impulsive nor sensation-seekers. Aust N Z J Psychiatry. 1988; 22: 307-311. ↵
- Gerra G, Angioni L, Zaimovic A et al. Substance use among high-school students: relationships with temperament, personality traits, and parental care perception. Subst Use Misuse. 2004; 39: 345-367. ↵
- Maremmani I, Pacini M, Popovic D et al. Affective temperaments in heroin addiction. J Affect Disord. 2009; 117: 186-192. ↵
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997; 5: 256-262. ↵
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999; 128: 78-87. ↵
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003; 11: 18-25. ↵
- Bornovalova MA, Lejuez CW, Daughters SB, Zachary Rosenthal M, Lynch TR. Impulsivity as a common process across borderline personality and substance use disorders. Clin Psychol Rev. 2005; 25: 790-812. ↵
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006; 60: 515-522. ↵
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007; 32: 950-966. ↵
- Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, Zvolensky MJ. The association between heroin use and anxiety sensitivity among inner-city individuals in residential drug use treatment. Behav Res Ther. 2006; 44: 667-677. ↵
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004; 99: 461-471. ↵
- Bornovalova M, Daughters S, Hernandez G, Richards J, Lejuez C. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Experimental and Clinical Psychopharmacology. 2005; 13: 311-318. ↵
- Lejuez CW, Bornovalova MA, Daughters SB, Curtin JJ. Differences in impulsivity and sexual risk behavior among inner-city crack/cocaine users and heroin users. Drug Alcohol Depend. 2005; 77: 169-175. ↵
- Zuckerman M. Sensation seeking and the endogenous deficit theory of drug abuse. NIDA Res Monogr. 1986; 74: 59-70. ↵
- Franques P, Auriacombe M, Tignol J. [Addiction and personality]. Encephale. 2000; 26: 68-78. ↵
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000; 68: 818-829. ↵
- Terracciano A, Lockenhoff CE, Crum RM, Bienvenu OJ, Costa PTJ. Five-Factor Model personality profiles of drug users. BMC Psychiatry. 2008; 8: 22. ↵
- Adams JB, Heath AJ, Young SE, Hewitt JK, Corley RP, Stallings MC. Relationships between personality and preferred substance and motivations for use among adolescent substance abusers. Am J Drug Alcohol Abuse. 2003; 29: 691-712. ↵
- Zuckerman M. Sensation seeking and the endogenous deficit theory of drug abuse. NIDA Res Monogr. 1986; 74: 59-70. ↵
- Franques P, Auriacombe M, Tignol J. [Addiction and personality]. Encephale. 2000; 26: 68-78. ↵
- Terracciano A, Lockenhoff CE, Crum RM, Bienvenu OJ, Costa PTJ. Five-Factor Model personality profiles of drug users. BMC Psychiatry. 2008; 8: 22. ↵
- Gossop M. Drug dependence, crime and personality among female addicts. Drug Alcohol Depend. 1978; 3: 359-364. ↵
- Labouvie EW, McGee CR. Relation of personality to alcohol and drug use in adolescence. J Consult Clin Psychol. 1986; 54: 289-293. ↵
- Greene RL, Adyanthaya AE, Morse RM, Davis LJ. Personality variables in cocaine- and marijuana-dependent patients. JpersAssess. 1993; 61: 224-230. ↵
- Clapper RL, Martin CS, Clifford PR. Personality, social environment, and past behavior as predictors of late adolescent alcohol use. J Subst Abuse. 1994; 6: 305-313. ↵
- Schinka JA, Curtiss G, Mulloy JM. Personality variables and self-medication in substance abuse. JpersAssess. 1994; 63: 413-422. ↵
- Ball SA, Kranzler HR, Tennen H, Poling JC, Rounsaville BJ. Personality disorder and dimension differences between type A and type B substance abusers. J Pers Disord. 1998; 12: 1-12. ↵
- Conway K, Swendsen JD, Rounsaville BJ, Merikangas KR. Personality, drug of choice, and comorbid psychopathology among substance abusers. Drug Alcohol Depend. 2002; 65: 225-234. ↵
- Gerra G, Bertacca S, Zaimovic A, Pirani M, Branchi B, Ferri M. Relationship of personality traits and drug of choice by cocaine addicts and heroin addicts. Subst Use Misuse. 2008; 43: 317-330. ↵
- Khantzian EJ, Mack JE, Schatzberg AF. Heroin use as an attempt to cope: clinical observations. AmJPsychiatry. 1974; 131: 160-164. ↵
- Khantzian EJ. Addiction: self-destruction or self-repair? JsubstAbuse Treat. 1989; 6: 75. ↵
- Khantzian EJ. Self-regulation factors in cocaine dependence--a clinical perspective. NIDA ResMonogr. 1991; 110: 211-226. ↵
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. HarvRevPsychiatry. 1997; 4: 231-244. ↵
- Khantzian EJ. Psychiatric illness in drug abusers. NenglJMed. 1980; 302: 869-870. ↵
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry. 1996; 66: 17-31. ↵
- Skinstad AH, Swain A. Comorbidity in a clinical sample of substance abusers. Am Jdrug Alcohol Abuse. 2001; 27: 45-64. ↵
- Gum AM, Cheavens JS. Psychiatric comorbidity and depression in older adults. Curr Psychiatry Rep. 2008; 10: 23-29. ↵
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry. 1996; 66: 17-31. ↵
- O’Brien CP. A Range of Research-Based Pharmacotherapies for Addiction. Science. 1997; 278: 66-70. ↵
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985; 142: 1259-1264. ↵
- O’Brien CP. A Range of Research-Based Pharmacotherapies for Addiction. Science. 1997; 278: 66-70. ↵
- Franques P, Auriacombe M, Tignol J. [Addiction and personality]. Encephale. 2000; 26: 68-78. ↵
- Franques P. Sensation seeking as a common factor in opioid dependent subjects and high risk sport practicing subjects. A cross sectional study. Drug and Alcohol Dependence. 2003; 69: 121-126. ↵
- Gunnarsdottir ED, Pingitore RA, Spring BJ et al. Individual differences among cocaine users. Addict Behav. 2000; 25: 641-652. ↵
- Kreek M, Laforge K, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002; 1: 710-726. ↵
- Swadi H. Individual risk factors for adolescent substance use. Drug and Alcohol Dependence. 1999; 55: 209-224. ↵
- Batts K, Grabill T, Galvin D, Schlenger W. Contextual and other factors related to workplace-based substance abuse prevention and earl intervention for adolescents and young adults [dissertation]. 2004. ↵
- Khantzian EJ. A contemporary psychodynamic approach to drug abuse treatment. AmJDrug Alcohol Abuse. 1986; 12: 213-222. ↵
- Jessor R, Jessor S. A social-psychological framework for studying drug use. NIDA Res Monogr. 1980; 30: 102-109. ↵
- Jessor R, Chase JA, Donovan JE. Psychosocial correlates of marijuana use and problem drinking in a national sample of adolescents. Am J Public Health. 1980; 70: 604-613. ↵
- Bartzokis G, Beckson M, Lu PH et al. Increased CSF volumes are associated with diminished subjective responses to cocaine infusion. Neuropsychopharmacology. 2000; 23: 468-473. ↵
- Bartzokis G, Beckson M, Lu PH et al. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000; 98: 93-102. ↵
- Bartzokis G, Beckson M, Lu PH et al. Cortical gray matter volumes are associated with subjective responses to cocaine infusion. Am J Addict. 2004; 13: 64-73. ↵
- Liu J, Liang J, Qin W et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009; 460: 72-77. ↵
- Tanabe J, Tregellas JR, Dalwani M et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009; 65: 160-164. ↵
- Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res. 2004; 1007: 10-18. ↵
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999; 156: 11-18. ↵
- Childress AR, Ehrman R, Wang Z et al. Prelude to Passion: Limbic Activation by “Unseen” Drug and Sexual Cues. PloS ONE. 2008; 3: e1506. ↵
- ↵
- Goldstein RZ, Alia-Klein N, Tomasi D et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009; 106: 9453-9458. ↵
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007; 54: 183-186. ↵
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002; 159: 1642-1652. ↵
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007; 144: 1153-1159. ↵
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999; 156: 11-18. ↵
- Childress AR, Ehrman R, Wang Z et al. Prelude to Passion: Limbic Activation by “Unseen” Drug and Sexual Cues. PloS ONE. 2008; 3: e1506. ↵
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Jneurosci. 2003; 23: 7839-7843. ↵
- Verdejo-Garcia A, Vilar-Lopez R, Perez-Garcia M, Podell K, Goldberg E. Altered adaptive but not veridical decision-making in substance dependent individuals. J Int Neuropsychol Soc. 2006; 12: 90-99. ↵
- Volkow ND, Fowler J, Wang G, Hitzemann R. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993; 14: 169-177. ↵
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatler J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001; 158: 2015-2021. ↵
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009. ↵
- Volkow ND, Fowler J, Wang G, Hitzemann R. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993; 14: 169-177. ↵
- Volkow ND, Fowler J, Wang G, Hitzemann R. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993; 14: 169-177. ↵
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2009. ↵
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007; 3: 4-16. ↵
- Tomasi D, Goldstein R, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007; 155: 189-201. ↵
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-Induced Cocaine Craving: Neuroanatomical Specificity for Drug Users and Drug Stimuli. American Journal of Psychiatry. 2000; 157: 1789-1798. ↵
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry. 2006; 163: 359-361. ↵
- Yin H, Mulcare S, Hilário MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009; 12: 333-341. ↵
- Yin H, Knowlton B. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006; 7: 464-476. ↵
- Yin H, Zhuang X, Balleine B. Instrumental learning in hyperdopaminergic mice. NeurobiolLearnMem. 2006. ↵
- Everitt BJ., Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research Reviews. 2001; 36: 129-138. ↵
- Everitt BJ., Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005; 8: 1481-1489. ↵
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ.. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behavioural Brain Research. 2009; 199(1): 89-102. ↵
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999; 398: 567-570. ↵
- Belin D, Everitt BJ. The Neural and Psychological Basis of a Compulsive Incentive Habit. In: Steiner H, tseng K, editors. Handbook of basal ganglia structure and function, 20. Elsvier, Academic Press; 2010. ↵

