2 Metric Conversion
Download this lab as a Microsoft Word document.
Download the lab response form (Microsoft Word document) to access the questions only.
Purpose
The purpose of this activity is to learn the common base measurements in the metric system and master metric conversion calculations.
Learning Objectives
At the conclusion of this exercise, students will be able to:
- Identify metric unit measurements.
- Convert from one metric quantity to another quantity.
- Convert from the imperial system to the metric system and vice versa.
Why It’s Relevant
There are only three countries (the United States of America, Myanmar, and Liberia) (Buchholz 2019) that officially use the imperial system of measurement, while the rest of the world uses the metric system. If you want to choose the computer with the most storage, work on your favorite German car brand, or know the temperature in Brazil, understanding the metric system is crucial. Not only is this measurement used across the world, it’s also simpler and easier to calculate than the imperial system. Additionally, American scientists use the metric system, scientific notation, and unit conversions, so measurements will be expressed in these formats throughout the semester.
Introduction
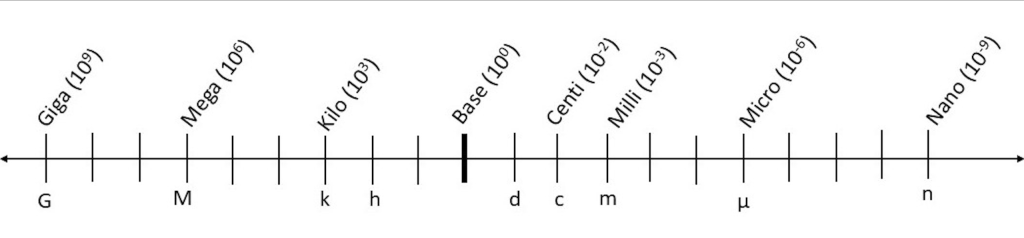
The metric system (also known as the International System of Units [SI]) is a system that is universally used in scientific inquiry. The metric system is a decimal system of weights and measurements in which the basic units are meter (m) for length, gram (g) for mass, and liter (l) for volume. The names of the units that are larger or smaller than the base unit use prefixes (originating from the Greek language), which are added to the base name (Table 2.1 and Figure 2.1). For example, kilo- means 1000, so 1 kilogram (kg) is equivalent to 1000 grams (g).
|
Common Metric Prefixes |
Symbol |
Measure relative to base unit |
Scientific notation |
Example in meters |
Example in grams |
Example in liters |
|---|---|---|---|---|---|---|
|
Giga |
G |
1,000,000,000. 00 |
109 |
Walking the equator of the earth about 8 times |
|
|
|
Mega |
M |
1,000,000.00 |
106 |
|
A very large commercial-grade refrigerator |
About ½ of an Olympic swimming pool |
|
Kilo |
k |
1000.00 |
103 |
Little over ½ a mile |
A bag of sugar |
3 standard bathtubs |
|
Hecto |
h |
100.00 |
102 |
Football (soccer or American) field |
A large orange |
|
|
|
Base Unit (meter, gram, liter) |
1.00 |
100 |
Baseball bat |
A single grain of rice |
A carton of milk/juice |
|
Deci |
d |
0.10 |
10-1 |
Pencil |
|
|
|
Centi |
c |
0.01 |
10-2 |
Dice |
A housefly |
|
|
Milli |
m |
0.001 |
10-3 |
A sharp pencil point |
A grain of salt |
A single drop from an eyedropper |
|
Micro |
µ |
0.000001 |
10-6 |
Bacteria |
A snowflake |
|
|
Nano |
n |
0.000000001 |
10-9 |
The length a fingernail grows in 1 second |
Drug delivery to specific cells (Patra et al. 2018) |
|
|
Pico |
p |
0.000000000001 |
10-12 |
|
|
|
Practice which metric unit (meters, grams, or liters) would be appropriate to use in the following scenarios:
1a. A medication pill/capsule:
1b. A cup of drinking water:
1c. The length of a microscope slide:
1d. The height of a building:
1e. The mass of the human body:
1f. A bottle of liquid shampoo:
Additionally, are the following quantities greater or less than the base unit?
2a. Kilogram (kg):
2b. Deciliter (dl):
2c. Micrometer (µm):
2d. Megaliter (Ml):
2e. Nanogram (ng):
2f. Gigameter (Gm):
Furthermore, when dealing with very large or very small numbers, measurements can be expressed in scientific notation. Scientific notation is a way to represent numbers in a more compact form by using significant digits multiplied by 10 raised to an appropriate power or exponent (Table 2.1). The exponent indicates how many places the decimal point should move. If the exponent is negative, the decimal point moves to the left, and if the exponent is positive, it moves to the right. For example,
7.2 x 10-4 = 0.00072
10-4 means the decimal point moves 4 times to the left.
Practice writing the following numbers in scientific notation:
3a. 1 =
3b. 0.03 =
3c. 4000 =
3d. 0.0000372 =
3e. 0.2 =
3f. 4,793,970 =
To measure large and small quantities, the unit cancellation method (also known as the ladder method, factor label method, dimensional analysis, stoichiometry in chemistry) is used to convert quantities within the metric system. The conversion equation is set up so that similar units are cancelled out, leaving the desired unit or factor. The equation can be set up in different ways. Below are some examples:
Convert 25 mm to cm (25 mm = ? cm)
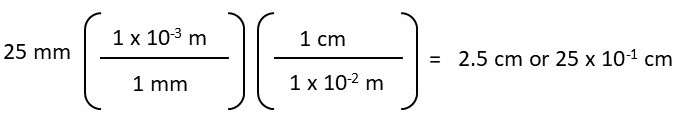
Or
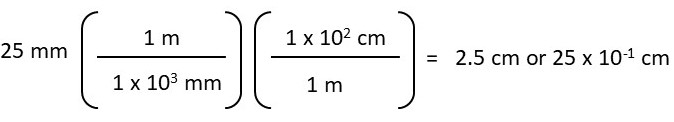
When the equation is set up correctly, the probability of making an error is lower compared to other unit conversion methods. To calculate the above conversion, you need to structure the equation so that “mm” cancels out, allowing you to convert to “cm.”
For example,
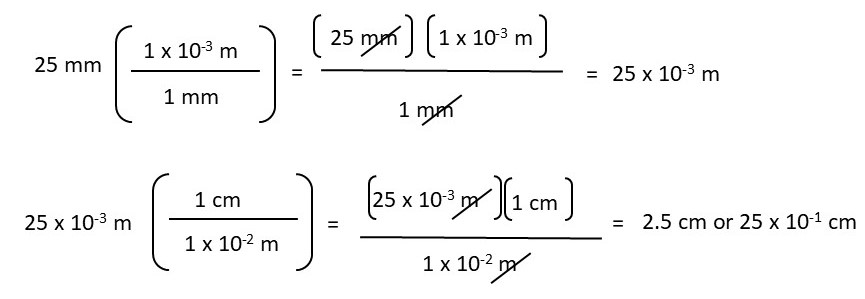
Practice using the unit cancellation method by performing the following metric conversions. Please show your work and write your answers in scientific notation.
4. 50 cm = ________ m
5. 4 x 10-1 kg = _______ g
6. 250 ml = _______ l
7. 3.5 x 103 g = _____ dg
8. 15 µl = ______ ml
9. 50 Gl = _______ l
10. 0.000732 nm = _______µm
11. 12 x 103 hg = ________ Mg
12. 300 µg = _______ g
13. 175 x 10-2 kl = __________ cl
Additionally, the unit cancellation method can be used to convert from the imperial system (also known as United States standard units) to the metric system. Below are common conversions between the imperial system and the metric system (Table 2.2):
|
Imperial Unit |
Approximate Metric Unit |
|---|---|
|
1 inch (in) |
2.54 cm |
|
1 yard |
91.44 cm |
|
1 mile |
1.61 km |
|
1 teaspoon (tsp) |
5.69 g |
|
1 tablespoon (tbsp) |
17.07 g |
|
1 pound (lb) |
453.59 g |
|
1 fluid ounce (fl oz) |
29.57 ml |
|
1 cup |
236.59 ml |
|
1 pint |
568.26 ml |
|
1 gallon |
3.78 l |
To convert from imperial to metric (or vice versa), you can use the same method as above. In other words, you need to structure the equation so that the known measurement cancels out, allowing you to convert to the unknown measurement.
For example:
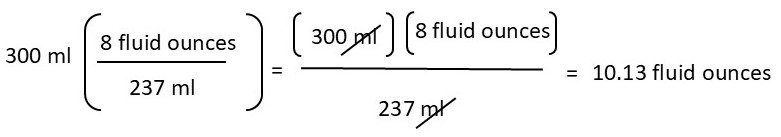
In future lab activities, you will convert from the metric to the imperial system (or vice versa) as needed. With this in mind, practice converting from the imperial system to the metric system and vice versa. Please show your work.
14. 15 in = _______ cm
15. 300 ml = ______ fluid oz
16. 7.93 g = _______ tsp
17. 10 gal = _______ l
18. 5 tbsp = ______ g
19. 140 lbs = _______ g
20. 25 miles = ______ km
Word problems
In the real world, it is rare that someone in your profession will ask you to calculate a conversion directly. Instead, they will typically ask for a specific measurement, such as “What is measurement A?” With that in mind, the most effective way to practice metric conversions is through word problems. Please show your work and write your answers in scientific notation when possible.
-
- Over-the-counter ibuprofen is typically 200 milligrams (mg) per pill. How many grams (g) is one pill?
- Many treatments for cancer carry the risk of damaging healthy tissue. However, nanotechnology drug delivery enables site-specific treatment, precisely targeting cancer cells within a tumor. Approximately 200 nanograms (ng) of nanoparticles (the drug) can accumulate in a tumor. How many grams of nanoparticles can be delivered into a tumor?
- Your laptop died, so you need to buy a new one. You use your laptop for school, and you also store your photos, music, and games on it. While you are shopping, you find laptops with the following hard drive sizes: 500 Gigabytes (GB), 700,000 Megabytes (MB), and 2 x 108 kilobytes (KB) for the same price. Which storage capacity is the best deal?
- A forensic scientist is investigating a murder case and collects hair evidence. A root of hair can provide 12 nanograms (ng) of deoxyribonucleic acid (DNA) (Bukyya et al. 2012). However, they need at least 0.025 micrograms (µg) of DNA to perform polymerase chain reaction (PCR) to amplify and analyze the DNA. How many roots of hair will the scientist need to perform PCR?
- The recommended dietary protein allowance for humans is 0.8 gram (g) per kilogram (kg) of body weight daily (Ryan-Harshman et al. 2006). If someone weighs 60 kg, how many decigrams (dg) of protein should they eat each day?
- One American football field (including the end zones) is 120 yards long. How many meters (m) is this? How many centimeters (cm)?
- How many miles do you have to commute to COD? How many km is this?
- The average gas tank capacity for a Toyota sedan is 45-55 liters. What is the gas tank capacity in gallons?
- Sara is studying abroad in Europe and craves chocolate chip cookies. Her famous recipe requires 5/8 of a teaspoon (tsp) of cinnamon. How many grams (g) of cinnamon will she need to purchase at the market?
- A Monster energy drink contains 86 milligrams (mg) of caffeine per 16-ounce can. How many tablespoons (tbsp) of caffeine will a person consume if they drink the whole can?
References
Buchholz. K 2019. Metric or Imperial? Statista [accessed 2025, Feb 8]. https://www.statista.com/chart/18300/countries-using-the-metric-or-the-imperial-system/
Bukyya, JL. et al. 2021. DNA profiling in forensic science: A review. Glob Med Genet. 8(4):135-143.
Ryan-Harshman, M. et al. 2006. New dietary reference intakes for macronutrients and fibre. Can Fam Physician. 52(2):177-179.
Patra, JK. et al. 2018. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16:71. https://doi.org/10.1186/s12951-018-0392-8
National Cancer Institute. [Internet] Cancer nanotechnology: treatment. Bethesda (MD): National Cancer Institute; [accessed 2025, Mar 16]. https://www.cancer.gov/nano/cancer-nanotechnology/treatment
University of California Museum of Paleontology. 2009. A billion years. [Internet]. Berkeley (CA): University of California Museum of Paleontology; [accessed 2025, Mar 16]. https://ucmp.berkeley.edu/education/explorations/tours/geotime/guide/billion.html
A decimal system of weights and measurements in which the basic units are meter (m) for length, gram (g) for mass, and liter (l) for volume.
A decimal system of weights and measurements in which the basic units are meter (m) for length, gram (g) for mass, and liter (l) for volume.
A word or letter placed before another word; A word before the base unit to measure quantity larger or smaller than the base unit.
A represent numbers by using significant digits multiplied by 10 raised to power or exponent.
A mathematical process used to converting measurements between units by systemically eliminating units in calculations.
A decimal system of weights and measurements that is historically used in the British Empire.

