11. The atomic nature of matter
Summary
Matter
It’s important to be able to categorize matter. From this categorization, we can learn about the various properties of different substances. We can also use this to determine what all matter has in common. Rather than being overwhelmed with the diversity of all of the different individual types of matter, we can realize that all matter can be placed into one of just a few categories. This categorization is outlined in Figure 11.1, and will be explained below.

Pure substances
All matter (that we are currently aware of) can be divided into two categories: pure substances and mixtures. In a pure substance, the properties of a substance such as density, melting point, specific heat capacity, and so on, are inherent to the material. A pure substance has a chemical composition that is known and does not change. For example: table salt (NaCl, or sodium chloride) is a pure substance. Water (H2O) is another pure substance with known chemical composition. However, salt water (a combination of water and an unknown amount of table salt) is a mixture (defined below), as the amount of salt added to the water can be varied.
Pure substances can be elements. Gold, aluminum, sulfur, and lead are examples of solid elements. Mercury is a liquid element. Oxygen, nitrogen, and neon are all gaseous elements. An element is a substance that consists of atoms whose nuclei have the same atomic number. An element cannot be broken down further by chemical means.
Pure substances can also be compounds, which are substances made out of many identical molecules (defined below). Water is a compound consisting of molecules. A water molecule itself consists of two hydrogen atoms and one oxygen atom.
Mixtures
In a mixture, the properties of the substance vary based on concentration. As mentioned above, salt water is a mixture. The boiling point, the density, and the freezing point are all examples of properties that vary depending on how much (and what type) of salt was used to create that salt water. A mixture consists of two or more pure substances that do not react chemically to form a new substance. As a result, the mixture can be separated into its constituent substances by physical means (such as filtering or distillation).
Mixtures can be either homogeneous or heterogeneous. Homogeneous mixtures, such as air, salt water, or even iced tea, are all uniform throughout their volume. When drinking a glass of iced tea, it doesn’t matter if you drink from the top or bottom of the glass, the iced tea will taste the same (it is homogeneous). Air is another homogeneous mixture. It doesn’t matter what part of the room you breathe in: the air is essentially the same everywhere in a room.
A heterogenous mixture does not have a uniform consistency throughout. Granite, for example (see Figure 11.2), is composed of different minerals. If you were to cut two pieces of the same size out of a piece of granite, each one might have different amounts of each mineral.

A more delicious example of a heterogeneous mixture is a burrito. Maybe you’ve had a burrito where one side is mostly lettuce, and in the middle is mostly sour cream, and the meat is all the way over to the other side. Depending on where you bite, you’re going to get a different taste! An example of a heterogeneous burrito mixture is shown in Figure 11.3.

Molecules
Molecules consist of two or more atoms that are held together by atomic bonds. A molecule is the smallest unit of a compound that still retains the identity of that compound. For example, water (H2O) is a molecule. If the water molecule is broken apart, it is no longer water – it will be hydrogen and oxygen.
Molecules can also be the building blocks of homogeneous and heterogeneous mixtures. Mixtures can be separated into two or more chemical substances. And those substances are made of molecules, which are made of atoms.
Atoms
Every solid, liquid, gas, and plasma is composed of atoms. For a long time, scientists thought that atoms were the smallest unit of matter. (The term “atom” comes from the Greek term “atomos,” meaning indivisible.) Although we now know that atoms are made out of smaller entities (protons, neutrons and electrons), the term atom has been retained.
Protons and neutrons are made of smaller entities called quarks. Electrons belong to a class of particles known as leptons. As of our current understanding, quarks and leptons are elementary particles, as described by the standard model of particle physics. The standard model is the theory we use to describe all known forces and elementary particles in physics. While far from being a complete and unified theory of everything, the standard model does a fantastic job explaining what we do know. The standard model predicted the existence of a particle called the Higgs boson. The discovery of this particle in 2012 gave further support for the validity of the standard model.
Atomism
Throughout time, humans have attempted to answer the question: what are the fundamental building blocks of matter? If we were to cut something in half, then in half again, and again, until we could do so no more, what would we find? Do all objects of matter share common building blocks, or is everything different?
Something of particular interest was whether matter was fundamentally discrete or continuous. That is: if we conducted the experiment where we continued to cut something in half until we could do so no more, would we find that there is a discrete particle, a building block of matter? This idea is known as atomism. Or would we find that there aren’t any discrete particles, but instead a continuum, some type of fluid or substance that fills the space it occupies?
Aristotle believed that the continuum model must be true of nature. The phrase “nature abhors a vacuum” is attributed to him. Aristotle believed that if matter was made of particles, between those particles must exist a vacuum – nothing. He argued that a vacuum would violate physical principles. Of course, Aristotle had no evidence to back up this philosophical assertion.
Scientists have since come to understand that atomism is the correct answer to the question of whether or not matter is fundamentally continuous or discrete. There are fundamental building blocks of matter (described by the standard model of particle physics), and those building blocks create atoms, out of which all matter is made.
Atomic models (discussed below) have provided scientific evidence supporting atomism. Before discussing them, it is possible to demonstrate atomism with an empty aluminum soda can and some gallium (a metal that has a very low melting point). Gallium becomes a liquid at 30 oC, which is slightly warmer than room temperature. In the demonstration, liquid gallium is poured on top of a soda can to see what happens after time passes.
Initially, the aluminum soda can is just a regular empty can. If you’ve ever drunk out of a soda can, you can get a feel for its strength. While it can be crushed with a sufficient amount of force, it’s a pretty strong container given how lightweight it is. In the video, before pouring gallium onto the can, Dr. Pasquale tests the strength of the can by pushing down on the top of the can with her fingers. Then, she pours liquid gallium onto the top of the can, placed near a space heater to keep the gallium in its liquid form. Dr. Pasquale then lightly scores the top of the can with a razor blade to expose the pure aluminum. After 20 minutes, the volume of gallium decreases as it diffuses into the can.
The can is now extremely weak and breaks apart as if it were tissue paper when Dr. Pasquale presses lightly with her fingers. The gallium inserted itself between the aluminum atoms and completely destroyed the bonds between each atom. If aluminum were made of a continuum, there would be no way for this to happen. Atomism is the correct interpretation of matter: all matter is made of atoms!
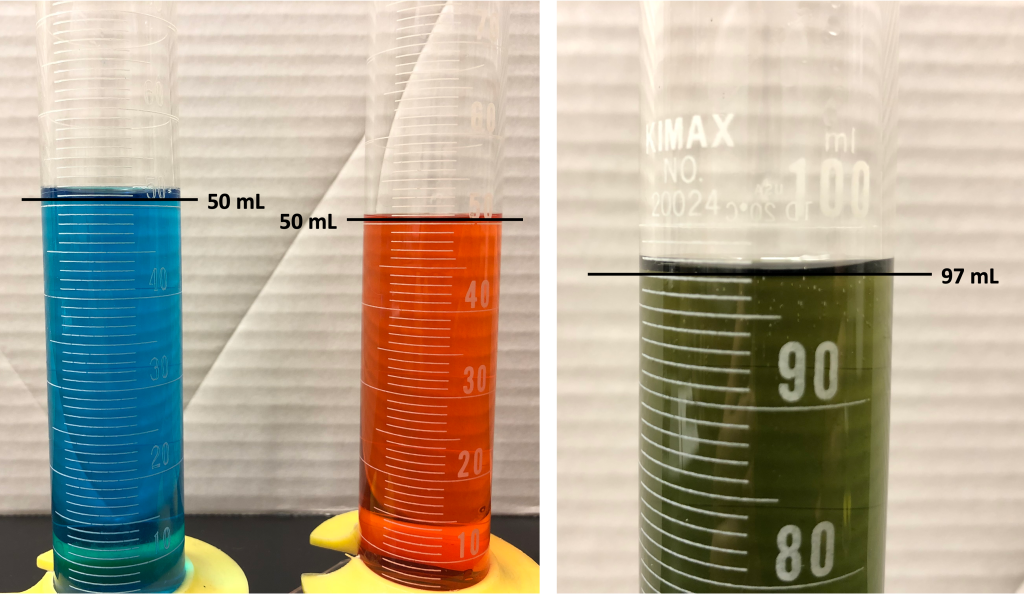
Another simple demonstration that supports atomism is to combine known volumes of water and alcohol. While the mass of this mixture is equal to the sum of the individual masses of the water and alcohol, the volume of the mixture will actually be less than the sum of the individual volumes! This is demonstrated in the video below.
Dr. Pasquale mixed 50 mL of water (dyed blue) with 50 mL of alcohol (dyed red), and the mixture of both liquids (a dark green color resulting in the mixtures of the food dyes) had a volume of 97 mL. These results are shown in Figure 11.4.
To understand why this is so, imagine a beaker filled halfway with marbles and another beaker filled halfway with BBs. When the marbles and BBs are mixed into a third beaker, the volume of the combination does not fill the entire beaker. This is demonstrated by Dr. Pasquale in the video below.
This result occurs because the smaller BBs can fill the gaps and voids between the larger marbles (see Figure 11.5). In a similar fashion, the smaller water molecules fill gaps and voids between the larger alcohol molecules.

Atomic theories
Many atomic theories have been proposed to explain the building blocks of matter: the atom.
Democritus, who lived around 400 BCE, theorized that everything was composed of atoms. Between atoms was empty space, and there were an infinite number of different types of atoms. He proposed that the properties of a substance related to the properties of the atoms that made it up. Iron would be composed of very strong interlocking atoms. Water atoms would be smooth and slippery. Salt atoms would be sharp and pointed. Many theories from this time period came about due to philosophical discussions and reasoning. It would be almost two thousand years before observations and experimental evidence were used in a systematic pursuit of scientific truth.
The scientist John Dalton, in the early 1800s, noticed that chemical compounds consisted of different elements in ratios of whole numbers. That is, things combined together in such a way that very consistent units of masses were involved. We now know this to be due to atomic bonds. A single oxygen atom can combine with two hydrogen atoms to create water. Water cannot be created with half an oxygen atom. There is no such thing as a half an atom, or a quarter of an atom. This seemed like a very convincing argument in favor of atomism.
Plum pudding model
In the late 1800s, J.J. Thomson discovered that the particles emanating from cathode ray tubes were much smaller than hydrogen, the lightest atom known. These particles are known to us today as electrons. Electrons were known at the time to create electric current. This discovery of electrons by themselves was evidence that atoms are not indivisible, but can be broken down into even smaller particles.
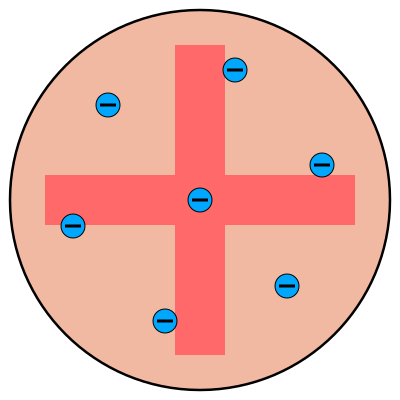
J.J. Thomson’s model, known as the plum pudding model, attempted to explain how atoms worked based on this knowledge. On the whole, it was known that atoms were electrically neutral. If they weren’t neutral, then all atoms would be attracted together or repelled apart due to electric forces. It was also known from the cathode ray tube experiments that electrons could move around by themselves. The plum pudding model hypothesized that an atom was made of a positively charged substance (pudding) that contained mobile electrons (plums). This is depicted in Figure 11.6. This model was eventually shown to be incorrect by Ernest Rutherford in the early 1900s.
Rutherford model
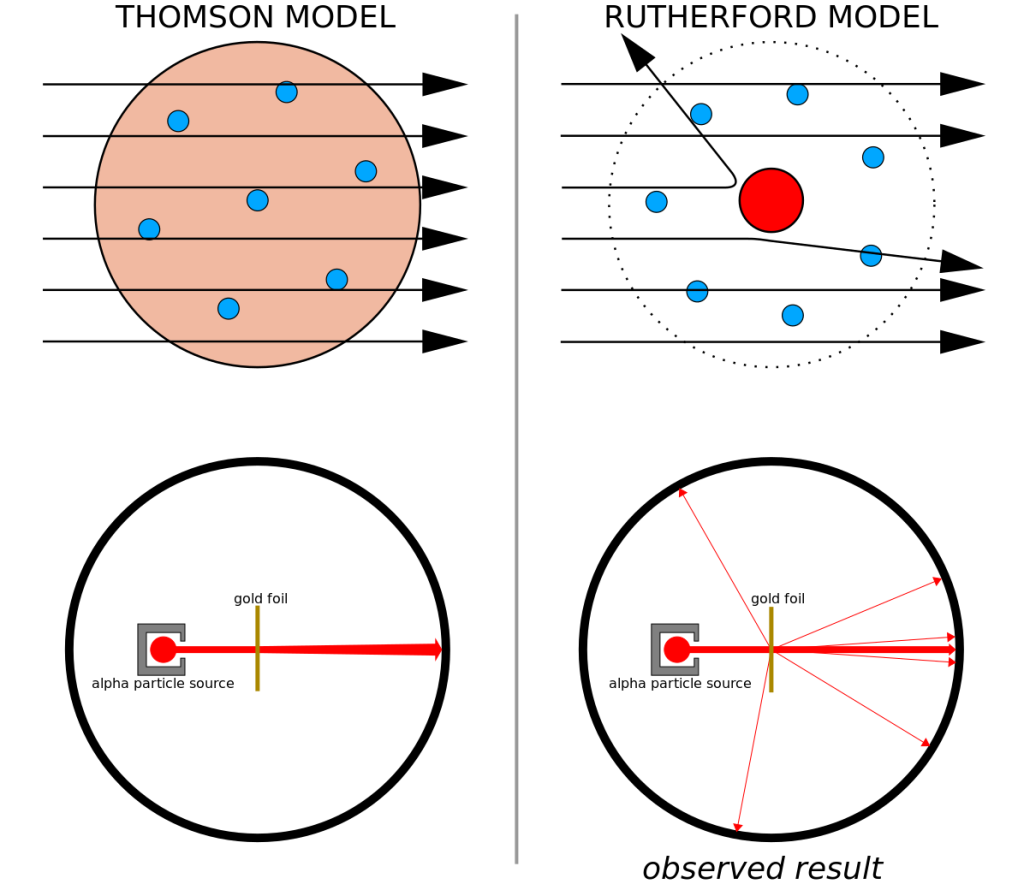
In the year 1911, Ernest Rutherford and his colleagues conducted an experiment where they shot alpha particles (electrically charged particles discovered by Rutherford in 1899) at a thin piece of gold foil to probe the structure of matter. They observed that the vast majority of the alpha particles passed through the thin piece of foil undeflected and only a small number were deflected. This outcome implied that most of the volume of gold foil is composed of empty space.
Of the small number of alpha particles that were deflected, the scattering angles were much larger than expected and a few bounced backward completely. To this, Rutherford stated: “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” This was a remarkable result because it meant that the alpha particles were rebounding off of a very massive yet very tiny object.
These unexpected experimental results disproved the plum pudding model proposed by J.J. Thompson. If the Thomson model had been correct, the expectation would be that all of the alpha particles would simply pass through the gold foil with some slight deflections (Figure 11.7, left).
Rutherford’s experiment led to the discovery of the atomic nucleus. This revised atomic model consisted of a very small positively charged nucleus surrounded by mostly empty space in which electrons reside (Figure 11.7, right). Alpha particles, we now know, are a helium nucleus consisting of two protons and two neutrons and having a positive electric charge. When they came to interact with the nuclei in the gold foil, they bounced back due to the force of electrical repulsion between the protons in the gold nucleus and the protons in the alpha particles.
Bohr (planetary) model
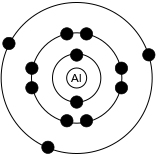
In 1913, Niels Bohr proposed that electrons orbit the nucleus of an atom much in the same way that planets orbit a star. These orbits were well defined, and only a finite number of orbits could exist. (In other words, only certain orbits were possible; an electron could not move around in any arbitrary orbit.) These orbits were defined by the amount of energy that electrons contained, which were measured by experiment as existing in very finite, discrete quantities.
The Bohr model is depicted in Figure 11.8, which shows an aluminum atom containing a nucleus and 13 electrons that orbit the nucleus in fixed orbits.
Current atomic model
What do we know about the atom today? Does the Bohr model stand the test of time? Based on the advances of quantum physics, we know that atomic models are not so straightforward. Electrons do not simply orbit the nucleus of an atom in circular or elliptical paths. Instead, they exist probabilistically. That means that we cannot know the exact location of an electron but can model the probability of where we expect an electron to be, given its energy level. In this way, we can describe electrons, and all subatomic particles, as “smeared out” rather than as single particles existing at a single point in space.
Eventually the neutron was discovered, completing our atomic picture. Neutrons are neutral, meaning they do not influence the charge of an atom, but do contribute to its mass. Because each atom is defined by the number of protons in its nucleus, atoms that have the same number of protons but different masses must contain different numbers of neutrons.
The periodic table
The periodic table of the elements (shown in a simplified form in Figure 11.9) is a table into which all elements are placed. The periodic table was developed over a span of many years and was proposed when scientist Dmitri Mendeleev noticed periodic repetitions of chemical properties of atoms.
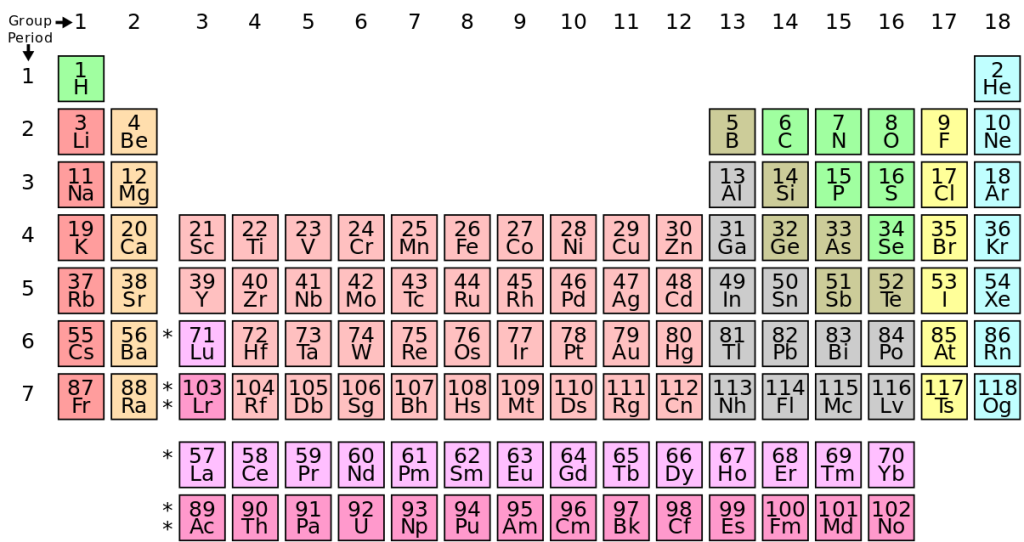
The columns on the periodic table represent elements that have similar chemical properties. Today we know that the columns correspond to the configuration of electrons in each element. For example, the column all the way to the right consists of noble gases. These gases do not readily react with other substances, and today we know this is because each of these elements contains a full shell of valence electrons.
The periodic table provides a lot of information about each element. Inside each box (for example, Oxygen is shown in Figure 11.10) is a symbol that is used to define the element in a chemical formula. Frequently, the name of the element will be spelled out as well. Oxygen has the symbol O. Lead has the symbol Pb.
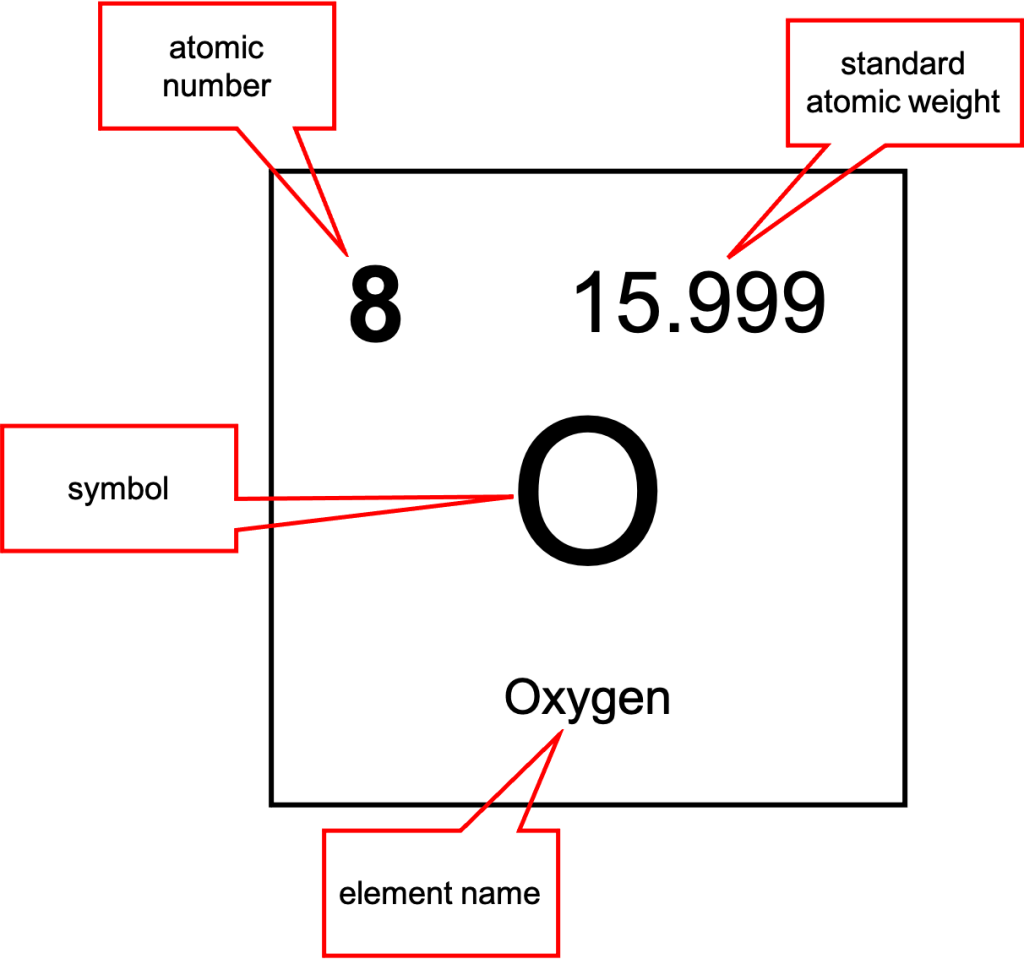
The number at the top left corner of each box is the atomic number of each element. The atomic number uniquely defines the element and is equal to the number of protons inside each atom’s atomic nucleus. We can therefore say that the number of protons is what defines each atom. Any atom that contains two protons is, by definition, a helium atom, regardless of how many neutrons or electrons are present.
Isotopes
While an element is defined by the number of protons (![]() ) in the nucleus, the number of neutrons (
) in the nucleus, the number of neutrons (![]() ) in the nucleus can vary. An atom with six protons is carbon. A large sample consisting only of carbon atoms will contain atoms that all have exactly six protons. Most of those carbon atoms also have six neutrons, but some have seven neutrons, and a few may even have eight neutrons. Elements that have different numbers of neutrons are known as isotopes.
) in the nucleus can vary. An atom with six protons is carbon. A large sample consisting only of carbon atoms will contain atoms that all have exactly six protons. Most of those carbon atoms also have six neutrons, but some have seven neutrons, and a few may even have eight neutrons. Elements that have different numbers of neutrons are known as isotopes.
Many elements have isotopes that occur naturally. Some are stable, and some are radioactive. Carbon-12 is the most abundant isotope of carbon. It is a stable isotope and is present in all organic matter. About 99% of all carbon atoms are carbon-12. Carbon-13 makes up most of the rest of carbon atoms. Carbon-14 is very rare (about 1 out of every one trillion carbon atoms) and is radioactive. This isotope of carbon is used in carbon dating. The ratio of carbon-12 and carbon-14 in an organic substance can be used to determine how old that substance is.
The standard atomic weight of an element is also given on the periodic table. The standard atomic weight of an element is the weighted average of the mass of all the naturally occurring isotopes of that element. This just means that the standard atomic weight takes into consideration the different isotopes of an element that exist, and the probability of finding each of those isotopes. This explains why the standard atomic weight given on the periodic table is usually not a whole number. When averaged over all of the natural isotopes, the result will generally not be an integer value.
Sometimes the standard atomic weight is provided in parenthesis, or not at all. This is when the number or abundance of isotopes is not yet known.
Other properties of an element are not going to be provided on the periodic table, but can be given to define an element. For example: ![]() describes helium-3. The number three is the atomic mass number (
describes helium-3. The number three is the atomic mass number (![]() ) of the isotope. Atomic mass number is equal to the number of nucleons (protons and neutrons) in the element. Because the element is helium, we know that it has two protons. Two protons plus the number of neutrons is equal to 3. Therefore, we can conclude that helium-3 has one neutron.
) of the isotope. Atomic mass number is equal to the number of nucleons (protons and neutrons) in the element. Because the element is helium, we know that it has two protons. Two protons plus the number of neutrons is equal to 3. Therefore, we can conclude that helium-3 has one neutron.
The atomic mass number can therefore be used to determine the number of neutrons in an isotope using the equation
![]()
Remember that the number of protons is given by the atomic number. The table below has several examples of different isotopes, including the number of protons and the number of neutrons in each isotope.
|
atom |
atomic number ( |
atomic mass number ( |
neutrons ( |
|
|
6 |
12 |
6 |
|
|
6 |
14 |
8 |
|
|
11 |
29 |
18 |
|
|
40 |
104 |
64 |
|
|
46 |
98 |
52 |
|
|
54 |
136 |
82 |
Ions
Atoms, on average, are electrically neutral. This means that the number of positively charged protons in an atomic nucleus is completely balanced by an equal number of negatively charged electrons orbiting that nucleus. When the number of protons and the number of electrons is no longer equal, due to an excess or absence of electrons, then the atom is charged. We call a charged atom an ion. The number of protons minus the number of electrons tells us the charge. A hydrogen atom has one proton. If a particular hydrogen atom has two electrons, then we know the charge of that atom is -1. The charge is represented as a superscript after the atomic symbol. This particular ion is represented as H–.
For example, an atom of ![]() is an atom of calcium. The number of protons (
is an atom of calcium. The number of protons (![]() ) is defined by the atomic number: 20. The atomic mass number is 40. To calculate the number of neutrons (
) is defined by the atomic number: 20. The atomic mass number is 40. To calculate the number of neutrons (![]() ), subtract the atomic number from the atomic mass number and obtain 20. There are 20 neutrons in the atom. Finally, the charge is +2. 20 protons minus the number of electrons equals +2. Therefore, there are 18 electrons in this ion of calcium.
), subtract the atomic number from the atomic mass number and obtain 20. There are 20 neutrons in the atom. Finally, the charge is +2. 20 protons minus the number of electrons equals +2. Therefore, there are 18 electrons in this ion of calcium.
What is the universe made of?
Is that all there is to the universe? Protons, neutrons, and electrons? Not exactly.
Antimatter
Antimatter is composed of particles included in the standard model of particle physics, and they have been observed by scientists. Antimatter particles have the same mass as normal matter, but the opposite charge. Protons have an antiparticle known as the antiproton, which is a negatively charged particle with the same mass as a proton. Electrons have an antiparticle known as the positron, which is a positively charged particle with the same mass as an electron. Neutrons also have an antiparticle known as the anti-neutron. When a particle and its antimatter partner come into contact, they annihilate each other and transform into radiant energy. The amount of energy transformed can be calculated using Einstein’s famous equation ![]() .
. ![]() is the energy generated,
is the energy generated, ![]() is the mass of the particles, and
is the mass of the particles, and ![]() is the speed of light.
is the speed of light.
Some antimatter is naturally produced, as the result of radioactive decay, for example. Antimatter is also artificially produced in particle colliders. These antiparticles take a lot of energy to create and only exist for a short time before getting annihilated by ordinary matter.
It appears pretty clear to scientists that our universe is composed almost entirely of what we consider to be normal matter, and not antimatter. Why is this? We don’t know. This asymmetry of matter and antimatter is one of the biggest unsolved questions in physics.
Dark matter and dark energy
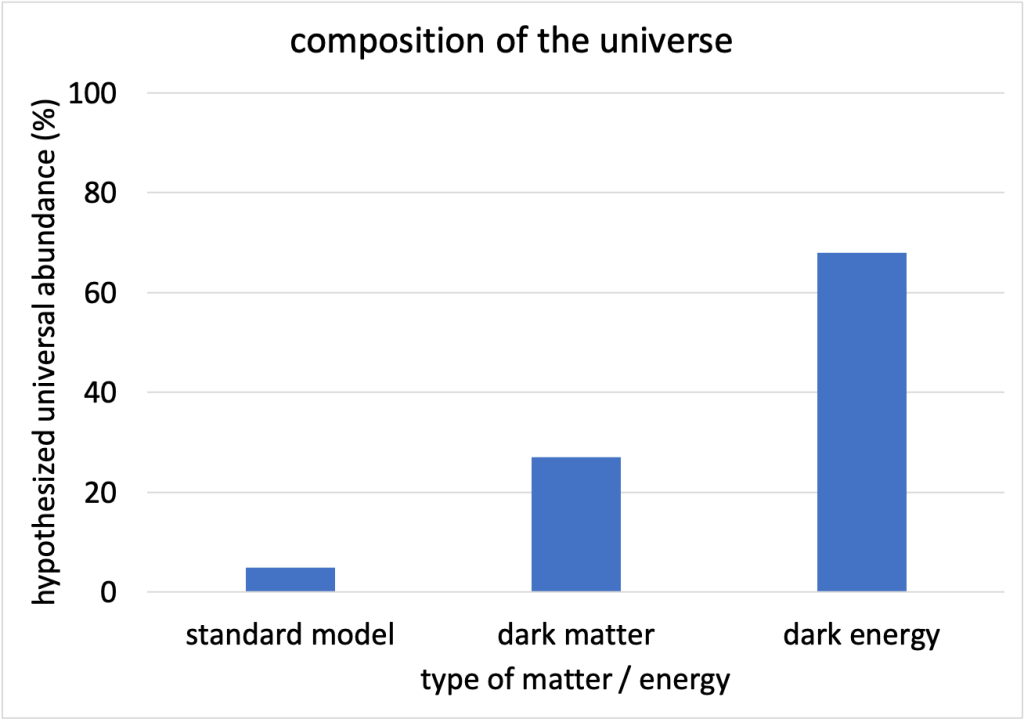
Many observations of the motions of galaxies indicate that they cannot be composed entirely of particles represented in the standard model of particle physics. In addition, observations about the rate of expansion of the universe cannot account for the amount of energy known to exist in the universe. In fact, the standard model probably only accounts for about 5% of the matter and energy that exist in the universe. Dark matter and dark energy are hypothesized concepts that enable the mathematics of the universe to work out correctly based on our observations. These concepts are called “dark” due to the fact we cannot “see” them directly. Rather, we infer their existence based upon the behavior of objects that we can see.
For instance, dark matter does not interact with electromagnetism. In other words, we cannot see dark matter because it does not interact with light. However, it does interact gravitationally, so we can observe the effects of dark matter from how it affects the motion of objects with mass that do interact with light.
The existence of dark energy has been proposed to explain the observation that the rate of expansion of the universe is increasing. The first evidence that the universe is expanding was presented by Edwin Hubble in 1929. This incredible discovery indicated that the universe appears to have had a “birth.” With this new picture of the universe, it was believed that the expansion of the universe should gradually slow down due to the gravitational attraction of all the matter in the universe. However, in the late 1990s, it was discovered that the rate of expansion was actually increasing! This means that either Newton’s law of universal gravitation breaks down at large-scale distances or that there is some other influence causing this observed “antigravitational” effect. The term dark energy has been assigned to explain this increasing rate of expansion of the universe.
It is hypothesized that 27% of our universe is composed of dark matter, and the remaining 68% is dark energy (see Figure 11.11). This is another area of intense research in physics today.
Further reading
- Gallium-induced structural failure of an aluminum baseball bat – Watch as the structure of aluminum is destroyed by gallium atoms that dissolve the aluminum bonds. This is a pretty good indication that matter is atomic; gallium would be unable to diffuse inside of the aluminum if it were a continuum.
- What and where is antimatter? – This short article from CERN explains some basics of antimatter and brings up some of the big questions about antimatter that physicists are trying to answer.
- Ptable.com – This website is a great interactive periodic table of the elements.
- Where Is Half of the Universe? – This article from The Physics Teacher discusses the imbalance of matter and antimatter in the universe.
Practice questions
Conceptual comprehension
- List five different mixtures. Which of those are homogeneous and which are heterogeneous? How do you know?
- List five different pure substances.
- What do the following atomic nuclei,
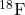 ,
, 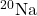 ,
, 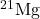 ,
, 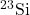 , have in common? (Hint: These are all the same “isotones.” Look up: what is an isotone.)
, have in common? (Hint: These are all the same “isotones.” Look up: what is an isotone.)
Numerical analysis
- How many protons are in the following elements?
- Hydrogen
- Helium
- Carbon
- Oxygen
- Iron
- Identify the number of protons in each of the following molecules.
- H2O (two hydrogen and one oxygen atom)
- CH4 (one carbon and four hydrogen atoms)
- N2 (two nitrogen atoms)
- CO2 (one carbon and two oxygen atoms)
- C6H12O6 (six carbon, twelve hydrogen, and six oxygen atoms)
- Identify the number of protons and the number of neutrons in each of the following atoms.
- Carbon-12
- Helium-4
- Oxygen-16
- Silicon-28
- Uranium-235
- Identify the number of protons, neutrons, and electrons in the following atoms.
Hands-on experiments
- Experiment with different substances like salt (sodium chloride) and sugar (sucrose). Add a small amount of each substance to a glass of water and stir until it dissolves. Note how the substances seem to disappear into the water, illustrating the concept of solubility and the dispersion of particles at the atomic level. You can then measure the density of each mixture to verify that the density varies with different concentrations of salt or sugar.
- Try the alcohol/water experiment for yourself. If you don’t have a graduated cylinder, use a measuring cup or measuring spoons to measure equal volumes of each liquid. Verify that the volume of the combined mixture is less than the individual volumes of each liquid.
In a pure substance, the properties of a substance (density, melting point, specific heat capacity, etc.) are inherent to the material.
Density describes the compactness of matter in a substance, defined as the mass per unit volume. (symbol: D, unit: kg/m^3)
Specific heat capacity defines how difficult it is to change the temperature of a substance. It describes the amount of heat required to change the temperature of a certain mass of a substance by a certain temperature. (symbol: c, unit: cal/(g°C))
An element is a substance that consists of atoms whose nuclei have the same atomic number. An element cannot be broken down further by chemical means.
A solid is a substance where the molecules or atoms are very tightly bound together. This gives a solid a very rigid volume and shape. Solid is one of the four most common phases of matter.
A liquid is a state of matter in which the constituent molecules will change their shape or arrangement but cannot be easily compressed to change their volume. Liquid is one of the four most common phases of matter.
Gas is a state of matter where molecules are very free to move about and generally do not interact with each other except during collisions. This means that the shape and volume of a gas is free to change. Gas is one of the four most common phases of matter.
The atomic number uniquely defines an element and is equal to the number of protons in atoms of that element. (symbol: Z)
A mixture is a substance where the properties of that substance (such as density, melting point, and specific heat capacity) vary based on the concentration of each component in the mixture.
Plasma is ionized gas. Plasma is the most common phase of matter in the universe.
A proton is a subatomic particle that has a positive charge and resides in the nucleus of an atom.
A neutron is a subatomic particle that has no charge and resides in the nucleus of an atom.
A quark is a fundamental building block of matter, described by the standard model of physics. It is the building block of protons and neutrons.
An electron is a fundamental building block of matter that has a negative charge and is found surrounding the nucleus of an atom.
A theory is an explanation of how the universe works that is supported by multiple, repeated experiments.
A force is a push or a pull that causes an object to change its motion. More fundamentally, force is an interaction between two objects. (symbol: F, unit: N)
Physics is a branch of science that focuses on the fundamentals of the workings of our universe.
Mass is a property of physical objects that relates to resistance to changes in motion: inertia. (symbol: m, unit: kg)
Current describes the flow of electric charge through a circuit. (symbol: I, unit: A)
Electric charge is a fundamental property of matter that causes particles that carry a charge to experience a force when in the presence of an electric field.
An alpha particle is a helium nucleus (consisting of two protons and two neutrons).
The orbit of a satellite describes its path around a planet or star.
The orbit of an electron describes the properties of the electron wave as it exists around the nucleus of an atom.
Energy is defined as the capability of an object (or collection of objects) to do useful work. (symbol: E, unit: J)
Quantum physics describes the physical laws used to describe the properties of matter and energy that occur on a very small scale.
Path describes the total distance that an object travels as it moves from one point to another, measured along its trajectory. It is a scalar quantity. (symbols: d, x [for horizontal path], y [for vertical path], unit: m)
Energy levels describe the quantity of energy that each electron orbiting an atom can have. Each atom is uniquely defined by its discrete energy level values. The lowest energy level is known as the ground state.
Elements that have the same number of protons but different numbers of neutrons are known as isotopes.
Radioactivity is the process by which an unstable nucleus releases energy and emits a particle.
The standard atomic weight is the weighted average of the mass of naturally occurring isotopes of the element.
Atomic mass number is equal to the number of nucleons (protons and neutrons) in an atom. (symbol: A)
An ion is a charged atom; there is an unequal number of protons and electrons in the atom.
Electromagnetism describes the interactions that occur between charged objects.

